Intermetallic Materials as Platforms for Electrocatalysis

The Hall group investigates chemical reactions catalyzed by solid surfaces to address critical challenges in renewable energy storage and conversion. Intermetallic materials, with their well-defined atomic structures, serve as an ideal platform for uncovering fundamental insights into electrochemical reactivity. These materials provide unparalleled control over both electronic properties and catalytic site ensembles, allowing independent fine-tuning of electronic effects and active site geometries. Such precision enables a deeper understanding of reaction mechanisms at the molecular level. This research not only advances our fundamental understanding but also facilitates the development of efficient, selective catalysts for transformative energy technologies.
Understanding the Electrode Microenvironment
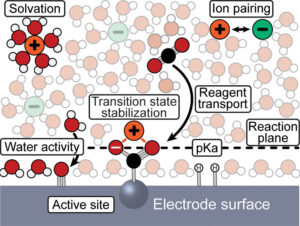
The electrode microenvironment is a dynamic zone where electrochemical transformations occur, comprising solvent molecules, ions, and the electrode surface, all of which govern reactivity and selectivity. The Hall group investigates these interactions at a fundamental level to control reaction outcomes and design interfaces that enable energy-efficient, selective processes like CO₂ reduction to multi-carbon products. Our primary focus lies in interfacial water, as its structure and activity critically influence reactivity in aqueous electrolytes.
Room Temperature Electrochemical Synthesis of Intermetallic Materials
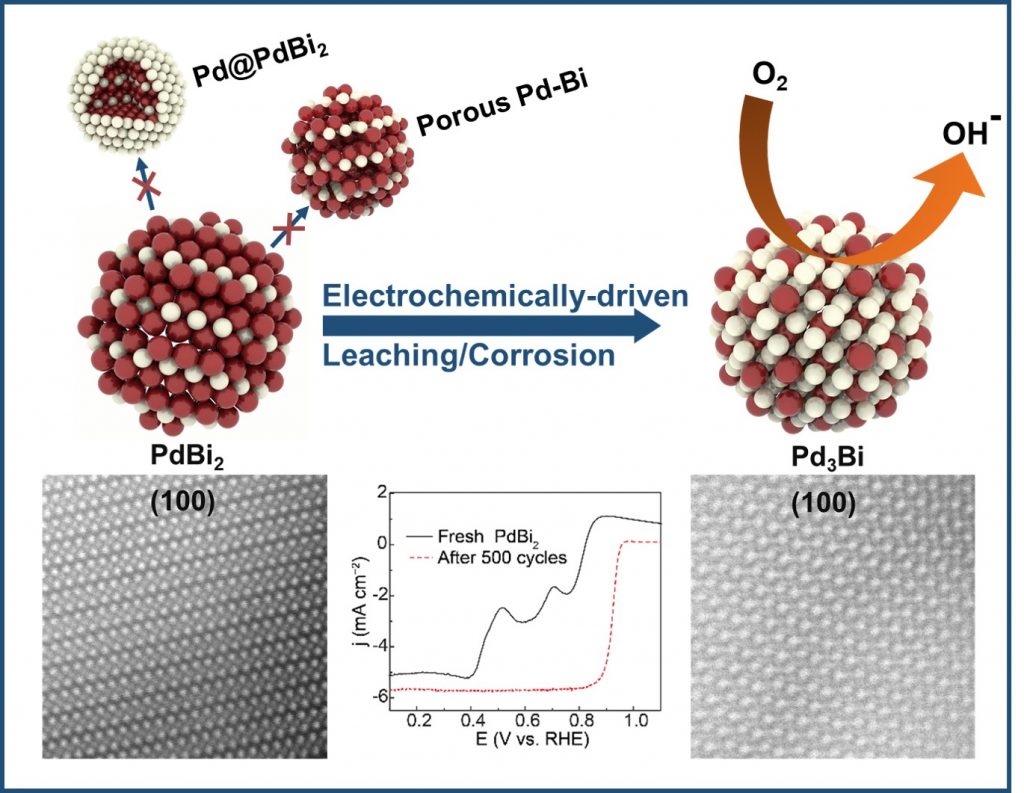
Intermetallic materials, alloys with long-range atomic ordering, exhibit exceptional properties for catalysis, electronics, and corrosion resistance. The Hall group is developing electrochemical methods (e.g., electrochemical dealloying, electrodeposition) to prepare nanostructured intermetallics at room temperature and atmospheric pressure. This approach addresses the challenges of conventional high-temperature methods, enabling precise control over composition and morphology. By identifying key trends, we aim to create high-performance materials with tailored properties, advancing the development of nanomaterials for cutting-edge applications.
